Quinone, Quinine, And Hydroquinone – What’s The Difference?
With names so strikingly similar, it’s no wonder these substances are easily confused with one another. So, let’s take a moment to sort through what makes quinone, quinine, and hydroquinone unique.

In this article, we’ll explain:
1. Quinones and aromaticity
2. From aromatics to quinones
3. Quinones in nature
4. Natural dyes and pigments
5. Quinine, it’s a drug
6. Gin and tonic anyone?
7. Fever Trees
8. Fluorescence
9. Hydroquinone, not a quinone at all!
10. REDOX, beetles and photography
11. More dyes
Quinones and aromaticity
Quinone, or more accurately quinones (since we are talking about a whole group of compounds not just a single chemical) are compounds that start life as aromatic compounds such as benzene or naphthalene. The term aromatic has a specific meaning in organic chemistry that goes way beyond just “smelly,” even though most aromatic compounds are indeed associated with strong aromas. The simplest definition of an aromatic compound is a cyclic molecule, i.e. one with a ring structure, that has alternating single and double carbon-carbon bonds. Such alternate single and double bonded arrangements in molecules are known as conjugated systems. There are more complex definitions of aromaticity, but using the examples of benzene and naphthalene below, one can see how these two molecules each fulfill our simplified parameters.
Benzene
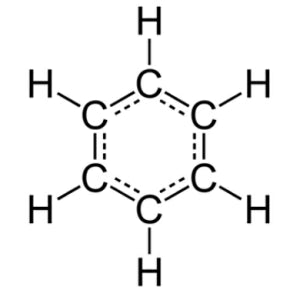
Naphthalene

From aromatics to quinones
So, having established what aromatic means and having seen what aromatic molecules look like, how do we get to quinones? The transformation is quite simple. Starting with an aromatic compound, the conversion of an even number of the original –CH= groups into –C(=O)– groups, plus necessary re-arrangement of carbon-carbon bonds, will yield a quinone. Applying those conversions to the two aromatics above, we get two quinones, each with two –CH= groups converted to –C(=O)– groups.
1,4-benzoquinone

1,4-napthaquinone

It’s worth noting a couple of things about these names. Firstly, the suffix (endings) of the names of these compounds has significance. The -one ending refers to the ketone group, i.e. a carbonyl group (a carbon atom double-bonded to an oxygen atom) with other carbon-containing groups flanking it on each side. In general,

Secondly, in common usage the singular ‘quinone’ is often used to refer to the singular compound 1,4-benzoquinone, even though the plural ‘quinones’ more accurately applies to the wider group of related compounds.
Quinones in nature
Quinones can be found extensively in naturally occurring organisms and have a wide range of functions. A few examples include ubiquinone, crucial to respiration, vitamin K1 (phylloquinone), and vitamin K2 (menaquinone) that are found in leafy greens and gut bacteria respectively. Anthraquinones are found in many plants that are natural laxatives like senna, and naphthoquinones are found in tea and coffee.
Natural dyes and pigments
Dyes and pigments have a rich history of being extracted from plants, and in several cases those florae have yielded quinone based compounds that are responsible for the colors. For example, lawsone (formally 2-hydroxy-1,4-naphthoquinone) is a red-orange compound found in the henna plant; from the madder plant, alizarin (formally 1,2-dihydroxyanthraquinone) has been used for thousands of years as a reddish-brown pigment.
Natural dyes and pigments
Quinine is a lot simpler to describe than quinones. Essentially it is the trivial name for a drug that has been used to treat malaria. Its IUPAC name is (8α,9R)-6′-Methoxycinchonan-9-ol , and it has the formula C20H24N2O2.

Gin and tonic anyone?
Perhaps more familiar is the presence of quinine in tonic water. It helps to impart that familiar bitter taste to the mixer, and in the past tonic water was advertised as being an anti-malaria treatment in and of itself.
Fever Trees
Quinine is a component of the bark of the cinchona tree found predominantly in South America. Because of the connection between quinine, the bark of the chinchona, and malaria, the tress that produced such bark have been called Fever Trees. It’s interesting to note that a leading brand of tonic water carries the same name.
Fluorescence
Quinine has a fascinating property known as fluorescence. When placed under an ultraviolet light the tonic water will appear to glow with a stunning, blue hue. The molecule has the ability to absorb light of one wavelength, and then emit light of a different one. The ability of a molecule to exhibit such a property is connected to its structure, with conjugated systems (those with alternate single and double bonds) being particularly prone to act in this way.
Hydroquinone, not a quinone at all!
The name hydroquinone refers to one specific compound that isn’t a quinone at all, but a phenol. Phenols are aromatic alcohols, i.e., compounds that contain -OH groups attached to aromatic (see above) compounds. Hydroquinone is a trivial moniker for the formally named 1,4-benzenediol, which has the formula C6H6O2, and the structure shown below. One can quite easily see the similarity both in name and structure between it and the aforementioned 1,4-benzoquinone.
1,4-benzenediol

REDOX, beetles and photography
Hydroquinone is readily converted to 1,4-benzoquinone in a REDOX reaction (one which involves oxidation and reduction). The hydroquinone is said to be oxidized, in this context by losing hydrogen atoms to produce 1,4-benzoquinone. Fascinatingly, this conversion happens in nature inside the bombardier beetle. The beetle (which can be found all over the world), has an interesting defense mechanism – it ejects a hot, noxious spray from its abdomen when it feels threatened. The reaction occurs between hydrogen peroxide and hydroquinone held inside the beetle, and it is a highly exothermic one, i.e. one that releases lots of energy. This creates the hot spray. In the reaction, hydroquinone is converted to 1,4-benzoquinone in a REDOX reaction, and in this context the hydroquinone is described as a reducing agent – meaning it has the ability to reduce other things.
In black and white photography, compounds of silver known as silver halides such as silver bromide, can be reduced to yield black silver particles and hence create an image. In order for this to happen, silver ions must be reduced to silver metal, and hydroquinone is used in the developing solution.
More dyes
In a connection to the dyes mentioned above, hydroquinone can be used to produce 1,4-dihydroxyanthraquinone also known as quinizarin or solvent orange 86. The orange-brown crystals can be used as a dye to color gasoline and oils.
In another example of its ability to change the color of things, hydroquinone was once used a skin lightener by removing dark colored age-spots but has since been banned in the USA over safety concerns.
Where to Buy Fireplace Bioethanol
As your trusted partner in chemical supply, Lab Alley is always striving to meet the growing demands of our customers. We want you to be able to access all of your chemical needs in one place, along with streamlined online ordering and fast shipping.
Due to popular demand from our valued clientele, Lab Alley has just added a new bioethanol product to our offerings. Our new bioethanol fuel is perfect for use in indoor and outdoor fireplaces.




Conclusion
Chemical waste disposal can be expensive for labs and businesses. Businesses must schedule waste pick-up from a hazardous waste disposal company. Minimizing your hazardous waste is one way to cut business costs. It will also reduce costs from replacing expired or spilled reagents.

