How To Dispose Of Sulfuric Acid And Clean Up Spills
Learn How To Safely Dispose Of Sulfuric Acid Waste
Dilute and neutralize H2SO4 to clean up spills in homes and labs. Read about the proper way to dispose of sulfuric acid, here.
Find out how to clean up sulfuric acid spills, here. Recommended procedures for chemical disposal are listed, here.
To neutralize sulfuric acid, pour it into a solution of sodium hydroxide. Use sodium bicarbonate (or baking soda) to neutralize battery acid (sulfuric acid).
Learn how to clean up sulfuric acid spills, here. Dispose of the spent sulfuric acid and disposables contaminated with sulfuric acid as hazardous waste.
Sulfuric acid can be disposed of by being placed in sealed containers and by being absorbed in vermiculite, dry sand, or earth.
How To Clean Up Sulfuric Acid Spills
For small sulfuric acid spills, cover the contaminated area with sodium bicarbonate or a mixture of soda ash/slaked lime (50/50) and mix.
Shovel the neutralized residues into containers for disposal. If neutralizing agent is not available, cover the area with sand or earth to absorb the liquid and shovel into containers for disposal
Sulfuric acid is listed as a toxic substance. Disposal of wastes containing sulfuric acid is controlled by a number of federal regulations.
It is not recommended that sulfuric acid or sulfur trioxide be placed in a landfill. Environmental regulatory agencies should be consulted for acceptable disposal practices.
Sulfuric acid has been disposed of by being placed in sealed containers and by being absorbed in vermiculite, dry sand, or earth. Sulfuric acid may also be diluted and then neutralized.
One method of neutralization is to add the acid slowly to a solution of soda ash and slaked lime, and to then flush with a large volume of water. Once sulfuric acid is diluted, and neutralized it can be discharged to a sewer.
When diluting, the acid should always be added to a large volume of water because the heat released when a small bolus of water is added can cause the water to turn to steam, and the resulting effervescence can splatter the acid.
Chemical Spill Response And Cleanup
Chemical Spill Response and Clean-Up
Uploaded to YouTube On March 21, 2012 by janette is cool
What To Do In Case Of A Sulfuric Acid Spill In Your Laboratory Workplace Or Home
Sulfuric Acid Spills And Exposure
If sulfuric acid is spilled or leaks and gets on your skin, quickly flush your skin with soap and lukewarm water for at least 30 minutes. Do not scrub or rub skin. If strong concentrations of gas or solution penetrate clothing, remove clothing and flush the skin with water. Seek medical attention immediately.
Immediately report leaks, spills or failures of the safety equipment (e.g. ventilation system). Prevent accidental contact with incompatible chemicals.
Sulfuric acid spills should be neutralized with sodium bicarbonate and then cleaned up with a paper towel or sponge.
-
Wait until bubbling/fizzing has stopped
-
When using a neutralizing spill kit, the kits are buffered and will not have a bubbling action. Be careful not to over-neutralize
-
Test pH of the spill after the neutralization reaction has stopped with pH paper
-
Once pH is between 6 and 9, the material can be transferred into an appropriate
-
secondary container for disposal
-
Wipe all surfaces with a sponge and wash all of the material down the sink.
Sulfuric Acid Safety, Videos, Storage And Disposal
-
Sulfuric Acid Usage: Learn how to take safety precautions.
-
Be careful when you buy, transport, use, handle, store and dispose of sulfuric acid solutions.
-
Buy sulfuric acid in safe containers, here.
-
Watch sulfuric acid safety videos, here.
Sulfuric Acid Safety Precautions

Sulfuric Acid Safety Tips
-
Take safety precautions to avoid sulfuric acid leaks and spills and wear acid resistant protective clothing.
-
Wear nitrile or natural rubber gloves for prolonged contact with sulfuric acid.
-
Learn how to use sulfuric acid safely at work or home by downloading and reading Safety Data Sheets (SDSs), here.
-
On this page, you can watch sulfuric acid safety videos and read informative OSHA sulfuric acid safety guidelines.
-
Learn how to use sulfuric acid in your home or at work without being harmed by this highly useful, but corrosive chemical.
-
If you or someone you are with has an exposure to sulfuric acid, call your local emergency number such as 911.
-
You can also contact your local poison control center can be reached directly by calling the Poison Help hotline, at 1-800-222-1222, from anywhere in the United States.
Use Sulfuric Acid Safe Containers For Transportation, Storage And Disposal

Transport sulfuric acid safely for use in pharmaceuticals, medicine, fertilizer, water treatment or car batteries. Buy sulfuric acid safe containers, sulfuric acid safe plastic bottles and jugs at Lab Alley, here.
Transport high concentrations of sulfuric acid in safe bottles or containers made from glass, polymethylpentene, polyethylene, teflon, viton or HDPE.
Mishandling this highly corrosive chemical can cause severe damage. Because this highly exothermic acid presents serious storage challenges, learn how to handle and store sulfuric acid it safely before you buy it.
Download a sulfuric acid handling and storage guide published by the Aetna Plastics company in PDF format, here.
Concentrated sulfuric acid products and solutions should be stored away from direct sunlight and heat sources in a cool, dry area.
Sulfuric Acid Shelf Life And Expiration Dates
Because sulfuric acid is an inorganic chemical, it has essentially an "infinite" shelf life. Some ACS grade sulfuric acid products do not have an expiration date. They will remain intact until it is reacted with other chemicals.
Sulfuric acid stays active for many years if it is stored properly. It is very stable and does not degrade or react unless it has contact with impurities or the atmosphere.
Lab Alley Brand Sulfuric acid has a very long shelf life and is normally stable. Reactivity hazards and conditions to avoid are water, humidity and moisture.
Sulfuric Acid FAQs
Q: What Happens If You Are Exposed To Sulfuric Acid?
A: Exposure to sulfuric acid may occur through skin contact, eye contact, ingestion, and breathing contaminated air. Severe exposure can result in death. Sulfuric acid can cause severe skin burns, it can burn the eyes, burn holes in the stomach if swallowed, irritate the nose and throat, and cause difficulties breathing if inhaled.
Q: What Should You Do If You Are Exposed To Sulfuric Acid?
A: If you get sulfuric acid get on your skin, immediately flush the contaminated skin with water. If you inhale sulfuric acid aerosols, seek fresh air and medical attention immediately. If liquid sulfuric acid or solutions containing sulfuric acid penetrate through the clothing, remove the clothing immediately and flush the skin with water. Get medical attention immediately.
How To Handle Sulfuric Acid Safely
Avoid generating sulfuric acid vapors or mists. Immediately address leaks, spills or failures of safety equipment.
Ingestion
If you ingest sulfuric acid, rinse your mouth immediately with water. Do not induce vomiting. Continually rinse your mouth with water and seek medical attention as soon as possible.
Inhalation
If you inhale sulfuric acid aerosols, seek fresh air and medical attention immediately.
Use sulfuric acid safely at work or in industrial settings by wearing the appropriate personal protection equipment (PPE), respirator, long rubber gloves, boots, industrial apron, chemical safety goggles and a face shield.
Sulfuric acid is commonly used for DIY and commercial household cleaning products and if it is not diluted, it is corrosive to metal and tissues.
Take safety precautions when you use sulfuric acid, (also known as oil of vitriol) for cleaning drains, janitorial and plumbing work, processing metal and making chemicals.
Sulfuric acid should not be stored indoors in large quantities, to prevent the possible accumulation of vapors.
Sulfuric Acid Respiratory Protection
People that work with sulfuric acid are required to wear respirators in various workplaces throughout the United States. Respirators protect workers against sulfuric acid, insufficient oxygen environments, harmful dusts, fogs, smokes, mists, gases, vapors, and sprays.
Sulfuric acid hazards may cause cancer, lung impairment, diseases, or death. Compliance with the OSHA Respiratory Protection Standard helps to prevent death and illness caused by sulfuric acid accidents.
What Do You Do If You Inhale Or Breathe In Sulfuric Acid?
Get medical attention immediately. If a person breathes in large amounts of sulfuric acid, move the exposed person to fresh air at once. If breathing has stopped, perform artificial respiration.
Sulfuric acid is a highly corrosive chemical that is potentially explosive in concentrated form. It can cause severe skin burns, can irritate the nose and throat and cause difficulties breathing if inhaled, can burn the eyes and possibly cause blindness, and can burn holes in the stomach if swallowed.
How To Treat A Sulfuric Acid Burn
The first and most important step is to dilute and get rid of all sulfuric acid at the burn site by irrigating profusely with water. Sulfuric acid will continue burning into the skin until it is removed. All clothing or equipment with sulfuric acid on it should also be removed by a person wearing protective gear. Read more here.
Sulfuric Acid Poisoning
Contact Poison Control Help right away if you suspect a sulfuric acid poising by calling 1-800-222-1222 and seek medical help right away.
Sulfuric acid is a very strong chemical that is corrosive. Corrosive means it can cause severe burns and tissue damage when it comes into contact with the skin or mucous membranes. Learn what to do in case of sulfuric acid poisoning from Mount Sinai, here.
Sulfuric Acid Chemical And Physical Properties
- Chemical formula: H2SO4
- Molar mass: 98.079 g/mol
- Appearance: Clear, colorless liquid
- Odor: Odorless
- Density: 1.8302 g/cm3, liquid[1]
- Melting point: 10.31[1] °C (50.56 °F; 283.46 K)
- Boiling point: 337[1] °C (639 °F; 610 K) When sulfuric acid is above 300 °C (572 °F; 573 K), it gradually decomposes to SO3 + H2O
- Solubility in water: miscible, exothermic
- Vapor pressure: 0.001 mmHg (20 °C)[2]
- Acidity (pKa): -2.8 , 1.99
- Conjugate base: Bisulfate
- Viscosity: 26.7 cP (20 °C)
Sulfuric Acid In The Workplace
Examples of How Sulfuric Acid is Applied in the Workplace
- Metal Processing
- Lead-Acid Battery Production
- Farm Harvests
- Pharmaceutical Manufacturing
How Sulfuric Acid Exposure Harms Workers
- Skin Contact
- Eye Contact
- Ingestion
- Inhalation
Best Safety Practices When Working with Battery Acid
- Handling
- Storage
- Clean-Up
What PPE Should Be Worn When Working with Battery Acid?
Overview Of Personal Protective Equipment From OSHA
Learn about sulfuric acid protective clothing in the OSHA Technical Manual, here. It covers, protective clothing selection factors, general guidelines, management program, clothing donning, doffing, and use, decontamination procedures, inspection, storage, and maintenance, training and risks.
Personal protective equipment, commonly referred to as "PPE", is equipment worn to minimize exposure to sulfuric acid that can cause serious workplace injuries and illnesses.
These injuries and illnesses may result from contact with chemical, radiological, physical, electrical, mechanical, or other workplace hazards. Personal protective equipment may include items such as gloves, safety glasses and shoes, earplugs or muffs, hard hats, respirators, or coveralls, vests and full body suits.
All personal protective equipment should be safely designed and constructed, and should be maintained in a clean and reliable fashion. It should fit comfortably, encouraging worker use. If the personal protective equipment does not fit properly, it can make the difference between being safely covered or dangerously exposed. When engineering, work practice, and administrative controls are not feasible or do not provide sufficient protection, employers must provide personal protective equipment to their workers and ensure its proper use. Employers are also required to train each worker required to use personal protective equipment to know:
-
When it is necessary
-
What kind is necessary
-
How to properly put it on, adjust, wear and take it off
-
The limitations of the equipment
-
Proper care, maintenance, useful life, and disposal of the equipment
If PPE is to be used, a PPE program should be implemented. This program should address the hazards present; the selection, maintenance, and use of PPE; the training of employees; and monitoring of the program to ensure its ongoing effectiveness.
Face Shield Protection From Sulfuric Acid
-
OSHA suggests that PPE such as face shield protection should be used as a last resort, as an engineering solution is preferred when working with sulfuric acid.
-
Engineering solution examples include using a chemical splash guard or a fume hood.
-
A chemical splash guard or a fume hood will stop sulfuric acid from ever reaching the face.
-
If an engineering solution is not practical, a face shield will offer limited chemical splash protection.
-
The manufacturer of the face shield is the best source for chemical-resistance data.
-
Buy Fisherbrand™ disposable and polycarbonate face shields for sulfuric acid, here.
Buy Sulfuric Acid PPE And Material Handling Equipment
Order Sulfuric Acid Personal Protective Equipment, Material Handling Equipment, Storage Containers And Transportation Equipment
Anyone who works in a wet chemistry lab, doing analysis and testing, needs a pair of safe sulfuric acid gloves, sulfuric acid splash goggles, and is some situations, a sulfuric acid face shield.
-
Buy sulfuric acid personal protective equipment (PPE) from 3M for worker health and safety, here.
-
Wear appropriate gear when you use sulfuric acid for industrial or scientific applications, gold recovery, pH adjustment, hair, batteries, pools, rust removal and toilets.
-
Find out what personal protective equipment (PPE) is needed when using sulfuric acid at home or at work, here.
-
Companies that sell sulfuric acid in bulk buy sulfuric acid tank trailers, here.
Sulfuric Acid Gloves
Sulfuric Acid Goggles
Sulfuric Acid Face Shields
Sulfuric Acid Respirators
-
Buy a Full Face Respirator from America Safety Associates (ADS), here.
-
Order a 3M Half-Mask Respirator from Grainger, here.
-
Get sulfuric acid respirator recommendations from the CDC, here.
-
Watch a YouTube video from the U.S. Department of Labor to learn about the different types of respirators, here.
-
Watch a YouTube video from the U.S. Department of Labor to learn about respirator safety, here.
Sulfuric Acid PPE Suits, Hoods, Coveralls, Jackets, Pants And Boots
-
Buy Made-To-Order Sulfuric Acid Safety Gear From The Standard Safety Equipment Company
-
Buy Sulfuric Acid Jackets And Sulfuric Acid Pants (Prices And Sizes)
Sulfuric Acid Pumps
Sulfuric Acid Tank Trailers
Heavy Duty Sulfuric Acid Handling And Loading Equipment
ChemSplash® 1 and ChemSplash® 2 Protective Suits
These industry leading product lines deliver reliable protection against skin burns caused by sulfuric acid splash and spills.
Coveralls and accessories have been rigorously tested to protect against noxious chemicals and come in styles with or without an attached hood and with or without attached boot coverings.
All chemical suits come with a storm flap over the zipper to prevent hazardous chemicals from seeping through exposed seams. Taped seams are also available for added protection against chemical splash.
Chemical splash aprons and sleeves are also offered to enhance safety and to decrease exposure areas on the body if a coverall is not worn.
Whether you need protection against light-duty chemicals, acids, and particulates or from more aggressive chemicals, acids, and caustics, the ChemSplash® product lines are fit for the job.
-
Gloves: Protective gloves are a necessity when handling even sealed sulfuric acid containers. A worker’s hands often make first contact with seepage or spilling liquid.
-
Face Mask: Routine protection calls for breathable masks to prevent inhalation. Traces of airborne acid can cause cancer or damage the lungs.
-
Face Shields: Clean-up crews and people handling open containers of sulfuric acid would be well-served to wear both a breathable mask and face shield.
Watch How-To Sulfuric Acid Demonstration Videos On YouTube
Sulfuric Acid Reactions
-
Use concentrated sulfuric acid (95-98%) and sulfuric acid 93% (92-94%) carefully because these concentrations react with water, sodium hydroxide, alcohol, sugar, aluminum, bases, metals and calcium carbonate.
-
Sulfuric acid reacts violently with alcohol and water to release heat.
-
Read a sulfuric acid safety guide, here.
- Watch a cool science demonstration video to see the power of concentrated sulfuric acid on YouTube.
Sulfuric Acid Occupational Safety Information
YouTube Video Titled "Sulfuric Acid Safety Information | Industrial Chemistry"
Uploaded by iitutor.com on March 14, 2016
Before working with sulfuric acid, individuals should be trained in its proper handling and storage and know how to use proper personal protective equipment, including protective gloves and chemical-resistant clothing and boots, splash-proof goggles, and respirators approved by the National Institute for Occupational Safety and Health (NIOSH) for use with sulfuric acid.
Use Sulfuric Acid Safely In Your Laboratory, Classroom Or Home
Pictured above are two amber brown glass bottles of concentrated sulfuric acid in a laboratory.
-
More sulfuric acid is produced in the United States every year than any other chemical.
-
Laboratory managers buy sulfuric acid, here. Buy sulfuric acid from Fisher Scientific, here.
-
Because this very strong chemical is so corrosive and reactive, it dissolves most types of metal, and unfortunately, human tissue as well.
- Purchase sulfuric acid gloves, here.
How To Handle, Store And Dispose Of Sulfuric Acid Safely
Contact With Sulfuric Acid, Burns, Exposure Symptoms And Treatments
-
A sulfuric acid burn is a medical emergency and requires immediate treatment.
-
If sulfuric acid makes direct contact with the eyes, it can cause permanent blindness.
-
Stay safe by learning about symptoms of sulfuric acid exposure from the CDC, here.
-
If you get sulfuric acid on you, flush your skin with soap and lukewarm water for at least 30 minutes. Do not scrub or rub your skin.
Personal Protective Equipment For Sulfuric Acid

PPE Required For Sulfuric Acid
-
It is important to use personal protective equipment, eye and face protection when working with sulfuric acid. Wear chemical safety goggles and a face shield when contact with H₂SO₄ is possible.
-
Protect your skin from sulfuric acid by wearing chemical-resistant protective clothing, gloves, an apron, boots and an NIOSH approved respirator.
-
Learn how to limit the risk of exposure to sulfuric acid in the workplace with the correct use of PPE (personal protective equipment), here.
-
Carelessness causes sulfuric acid damage, skin burns and injuries in workplaces and in homes when this corrosive chemical is splashed on an uncovered face or eyes.
-
Wear acid resistant protective clothing and gloves when you work with this corrosive substance in your laboratory, at your job or in your home.
Sulfuric and Hydrochloric Acid Safety Training Video Preview
YouTube Video Titled "Sulfuric and Hydrochloric Acid Safety Training Video"
Uploaded on February 12,2014 by SafetyVideos.com
This Sulfuric and Hydrochloric Acid safety video teaches fundamental lessons about identifying corrosive acids and the properties that make them hazardous to humans and the environment.
The video describes safe handling and storage of these chemicals, proper loading and off-loading procedures during transportation, and proper use of personal protective equipment and clothing.
The program also covers steps to take in the event of an uncontrolled spill, leak, or fire, including identifying the hazardous material involved, isolating the area, and assessing the dangers before taking action to control the accident.
This safety training video discusses how the reactivity of acids will affect materials used to seal leaking containers, and what protective clothing and other personal protective equipment to use.
Other topics covered include:
-
Emergency medical operations
-
Diking to control spills
-
Neutralization to control spills
-
Vapor control
-
Decontamination of persons and equipment following an acid spill or incident
-
Runoff water control
Hydrochloric and Sulfuric Acids have a destructive and irreversible effect on human tissue.
This program provides important safety information for workers who use, store or transport sulfuric or hydrochloric acid, as well as for emergency response personnel who might respond to an incident involving corrosives.
Give your affected employees the safety training they need with this important safety video. The runtime is 26 minutes and the video is available in DVD or VHS for $395, here.
Sulfuric Acid Workplace Health And Safety
-
Sulfuric acid is the largest volume chemical produced in the United States.
-
Concentrated sulfuric acid is extremely corrosive and dangerous to work with because it is destructive to the skin (corrosion and acute irritation), eyes, lungs (PDF), mucous membranes and teeth (tooth and dental erosion).
-
Sulfuric acid is a hazardous substance that causes chemical burns, injuries, poisonings, health problems and fatalities in workplaces located throughout the United States.
-
Outdoor workers are at risk of sulfuric acid exposure if they work in areas where coal, oil, or gas are burned.
-
Mechanics who handle dirty batteries are also at risk of sulfuric acid exposure.
-
Sulfuric acid is on the New Jersey Right to Know Hazardous Substance List (RTKHSL) because it is cited by OSHA, ACGIH, DOT, NIOSH, NTP, DEP, IARC, NFPA and EPA.
-
You can download a New Jersey Right To Know Hazardous Substance Fact Sheet For Sulfuric Acid in PDF format, here.
-
This sulfuric acid fact sheet contains information on health hazards, exposure limits, personal protective equipment, proper handling, first aid, and emergency procedures for fires and spills.
-
The chemical formula of sulfuric acid is H2SO4 and its molecular weight is 98.079 g/mol.
-
Sulfuric acid is on the Special Health Hazard Substance List (SHHSL) available in PDF format, here.
-
The New Jersey Right to Know Hazardous Substance List contains over 2,000 hazardous substances, including those on the Special Health Hazard Substance List (SHHSL).
-
The SHHSL consists of over 1,000 hazardous substances that are defined as carcinogens, mutagens, teratogens, corrosive, flammables, and reactives.
Buy Sulfuric Acid Gloves

Use Sulfuric Acid Gloves To Stay Safe
-
Grainger sells sulfuric acid gloves that have been tested and approved for use with sulfuric acid.
-
Always wear gloves when using a concentrated sulfuric acid solution in your at home or in workplace, because it can cause severe acidic chemical burns and even secondary thermal burns due to dehydration when they come into contact with skin and body tissues.
-
Dilute sulfuric acid, which is a strong acid and a good electrolyte, is substantially less hazardous but it should be handled with care for due to acidity.
How To Work With Sulfuric Acid Safely
Find out how to purchase, use, handle, store, transport, clean up and dispose of sulfuric acid with a minimum of risk.
Information on sulfuric acid safety and hazards is available from The National Institute for Occupational Safety and Health (NIOSH), here.
Sulfuric acid safety precautions must be understood and used because this corrosive chemical is destructive to the skin, eyes, teeth, and lungs. Severe exposure can even result in death.
Information on sulfuric acid laboratory hazards, sulfuric acid dilution hazards and sulfuric acid industrial hazards are listed, here.
Find out how sulfuric acid is used to produce other chemicals, as an industrial cleaning agent to remove oxidation, rust, and scaling from metals, here.
Learn how sulfuric acid is used as a catalyst in the chemistry industry, as an electrolyte in lead-acid batteries and as a drain cleaner, here.
The corrosiveness of sulfuric acid solutions is highly dependent on concentration, acid impurities and temperature.
Sulfuric acid (CAS Registry Number 7664-93-9), also known as hydrogen sulfate, is a highly corrosive, clear, colorless, odorless, strong mineral acid with the formula H2SO4. Download an occupational health guideline for sulfuric acid from the CDC, here.
Sulfuric Acid Respirator Pictured Below
Sulfuric Acid Respirators, Exothermic Reactions And Face Shields
-
What makes sulfuric acid so dangerous is its exothermic reaction. If you are going to be working with sulfuric acid, consider buying a 3M acid respirator, online here.
-
Sulfuric acid reacts vigorously with water in a highly exothermic reaction.
-
If you add water to concentrated sulfuric acid, it can boil and spit and you may get a nasty acid burn.
-
Learn about faceshield protection for sulfuric acid, here.
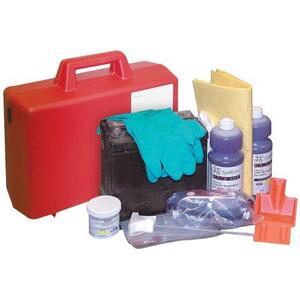
Sulfuric Acid Safety Precautions, Hazmat Training, Spill Kits And Gloves
December 17, 2021 - Pictured here are two spill kits in a work environment.
Sulfuric Acid Spill Kit Requirements And Components
-
Sulfuric acid (battery acid) spill kits include gear for battling this toxic corrosive.
-
The Occupational Safety and Health Administration standard that applies to spill kits is 1910.120, Hazardous Waste Operations and Emergency Response (HAZWOPER).
-
Required components are nitrile safety gloves, eye goggles, poly aprons, shoe or boot covers, polymers to neutralize acid, specialized scoops, disposal containers and a spill containment handbook.
Sulfuric Acid Spill Kit Pictured Below
December 17, 2021 - Pictured here is a battery acid (sulfuric acid) spill kit. Use this in garages, forklift battery storage areas workplaces to ensure a quick response to leaking or spilled sulfuric acid.
Sulfuric Acid In The Workplace
-
Companies often have hazardous materials such as sulfuric acid in their facility.
-
Sulfuric acid is often used in concentrated industrial drain cleaners and forklift batteries.
-
OSHA requires all employers to provide Hazard Communication training for new employees and additional training when new hazards enter the work force.
-
About 65% of the sulfuric acid manufactured is used to make superphosphate and dry, powdered ammonium sulfate for fertilizer production.
-
Because sulfuric acid is one of the strongest dibasic acids, concentrated solutions should be handled with gloves for sulfuric acid.
-
Liquid and gas-tight sulfuric acid personal protective equipment (PPE) and sulfuric acid PPE suits for biohazard chemical safety are highly recommended for protecting your skin when you are using sulfuric acid.
-
You can can buy disposable or reusable sulfuric acid PPE suits to protect yourself and your workers from this corrosive chemical.
Sulfuric Acid Chemical 3D Structure Model Depiction
Sulfuric Acid 2D Chemical Structure Depiction
Sulfuric Acid Chemical Properties, Hazards And Uses
- Sulfuric Acid Formula: H2SO4
- Sulfuric Acid CAS Number: 7664-93-9
- Sulfuric Acid Density: 1.83 g/cm³
- Sulfuric Acid Boiling Point: 638.6°F (337°C)
- Sulfuric Acid Melting Point: 50°F (10°C)
- Sulfuric Acid Molecular Weight: 98.079 g/mol
- Sulfuric Acid Index of Refraction: 1.537
- Sulfuric Acid Solubility In Water: Fully miscible; (exothermic)
- Sulfuric Acid Acidity (pKa): −3, 1.99
- Sulfurc Acid PubChem CID: 1118
- Sulfurc Acid ChemSpider ID: 1086
- Sulfuric Acid On ChemicalBook.com
- Sulfuric Acid Safety And Hazards
- Sulfuric Acid Structure, Properties, Spectra, Suppliers And Links
- Sulfuric Acid Uses: Industrial Production Of Chemicals, Sulfur–Iodine Cycle, Industrial Cleaning Agent, Catalyst, Electrolyte And Domestic Uses
Sulfuric Acid Health Hazards And Exposure Risks
If sulfuric acid makes direct contact with the eyes, it can cause permanent blindness. If ingested, this chemical may cause internal burns, irreversible organ damage, and possibly death. Sulfuric acid reacts violently, becoming very hot, when mixed with water (PDF).
Exposure to sulfuric acid aerosols at high concentrations leads to severe eye and respiratory tract irritation and tissue damage. For a 15-minute exposure, the vapor concentration of sulfuric acid in the atmosphere should not exceed 0.15 3 mg/m3.
Contact with sulfuric acid can cause pain, redness, burns and blistering. Permanent scarring can result. A severe exposure can cause death. Further testing of sulfuric acid (PDF) is required to assess its potential to cause reproductive harm.
Sulfuric Acid Is Corrosive To All Body Tissues
Sulfuric acid is extremely corrosive to all body tissues. It is corrosive and irritating and causes direct local effects on the skin, eyes and gastrointestinal tracts after direct exposure.
It causes rapid tissue destruction and serious chemical burns on contact with the skin or eyes. Skin or eye contact requires immediate first aid. Inhalation of sulfuric acid mist or fumes may produce irritation of the nose, throat and respiratory tract.
Identification Of Sulfuric Acid
Sulfuric acid is a dense, oily liquid that can be colorless to brown, depending on the purity. It can also exist as ice- or fiber-like crystals or as a gas. Sulfuric acid is also called battery acid. It is odorless with a strong acid taste. Sulfuric acid reacts violently with water, generating much heat. It is highly corrosive. Read more here.
Sulfuric acid is very irritating and corrosive to the skin, eyes, respiratory track and gastrointestinal track. Ingestion of sulfuric acid can burn the mouth and throat, and erode the stomach; death can occur. Direct eye contact can result in blindness. High concentrations in air may make it difficult to breathe, especially for those with asthma or during strenuous exercise. Chronic lung disease (bronchitis, fibrosis, emphysema), reduced lung function, and tooth decay have been reported occurred following occupational exposure to sulfuric acid. Increased tumors in the respiratory tract (nasal passages, larynx, lung) have been associated with occupational exposure to sulfuric acid in various industries. These studies are limited by co-exposure to several other workplace chemicals and/or tobacco smoke; however, the large number of studies reporting tumors suggests that sulfuric acid is a carcinogen. Data on the potential for sulfuric acid to cause infertility, abortion, or birth defects in humans were not available. No evidence for increased abortion or birth defects were observed in laboratory animals that breathed moderate levels of sulfuric acid during pregnancy. Data on the potential for sulfuric acid to cause infertility in laboratory animals were not available. The International Agency for Research on Cancer and the U.S. National Toxicology Program 13th Report on Carcinogens determined that occupational exposure to strong inorganic mists containing sulfuric acid is carcinogenic to humans, based on sufficient evidence of carcinogenicity from studies in humans. The potential for sulfuric acid to cause cancer in humans has not been assessed by the U.S. EPA IRIS program. Read more here.
How Dangerous Is Sulfuric Acid?
Sulfuric acid is a highly corrosive chemical that is potentially explosive in concentrated form. It can cause severe skin burns, can irritate the nose and throat and cause difficulties breathing if inhaled, can burn the eyes and possibly cause blindness, and can burn holes in the stomach if swallowed. Read more here.
Sulfuric Acid Is Dangerous | Hazards And Safety Information
If sulfuric acid makes direct contact with the eyes, it can cause permanent blindness. If ingested, this chemical may cause internal burns, irreversible organ damage, and possibly death. Exposure to sulfuric acid aerosols at high concentrations leads to severe eye and respiratory tract irritation and tissue damage.
Sulfuric acid is a highly corrosive chemical that is potentially explosive in concentrated form. It can cause severe skin burns, can irritate the nose and throat and cause difficulties breathing if inhaled, can burn the eyes and possibly cause blindness, and can burn holes in the stomach if swallowed.
Sulfuric acid is corrosive to all body tissues. Inhalation of vapor may cause serious lung damage. Contact with eyes may result in total loss of vision. Skin contact may produce severe necrosis. Fatal amount for adult: between 1 teaspoonful and one-half ounce of the concentrated chemical. Even a few drops may be fatal if the acid gains access to the trachea.
Yes Sulfuric acid can kill you. Here is info about sulfuric acid poisoning. How well a patient does depends on how fast the poison is diluted and neutralized. Extensive damage to the mouth, throat, eyes, lungs, esophagus, nose, and stomach are possible. Read more here.
What To Do If You Get Sulfuric Acid On Your Skin
Flush skin that was contaminated with sulfuric acid with soap and lukewarm water for at least 30 minutes. Do not scrub or rub skin. If strong concentrations of gas or solution penetrate clothing, remove clothing and flush the skin with water.
Read And Download PDFs Of Sulfuric Acid Risks In Safety Data Sheets (SDSs)
Because sulfuric acid is very corrosive (HAZMAT Class 8), learn how to avoid getting burned or killed, by reading about basic sulfuric acid safety precautions and how to use personal protective equipment (PPE).
Download Safety Data Sheets (formerly MSDS) for various grades and concentrations of sulfuric acid.
- Lab Alley Brand Sulfuric Acid 96% ACS Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 92-94% Lab Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 70% Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 50% Lab Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 40% Analytical Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 39% Technical Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 25% Analytical Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 20% Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 10% Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 6% Analytical Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 5% SDS (PDF)
- Lab Alley Brand Sulfuric Acid 1.5% Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 0.95N Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 0.2N Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 5N SDS (PDF)
- Lab Alley Brand Sulfuric Acid 1N Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 2N SDS (PDF)
- Lab Alley Brand Sulfuric Acid 0.1N Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 0.02N for Alkalinity, Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 0.5N Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 1:1 Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 19.2N Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 0.053N Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid Gerber/Babcock Analytical Reagent Grade SDS (PDF)
- Lab Alley Brand Sulfuric Acid 0.02N (n/50) Reagent Grade SDS (PDF)
- Teck Sulphuric Acid Safety Data Sheet (PDF)
- Sulfuric Acid 3M Safety Data Sheet - Fisher Scientific (PDF)
- Ashta Chemicals Safety Data Sheet Sulfuric Acid, Spent 70-80% (PDF)
- Avantor Performance Materials Sulfuric Acid Material Safety Data Sheet (PDF)
- Durham Tech Sulfuric Acid Safety Data Sheet (PDF)
- Sulfuric Acid Safety Data Sheet | CAMEO Chemicals | NOAA
- Sigma-Aldrich Sulfuric Acid Material Safety Data Sheet (PDF)
- JR Simplot Sulfuric Acid 98% Safety Data Sheet (PDF)